Swine IL-17A Polyclonal Antibody
IL-17A is a member of the IL-17 family, which is comprised of 6 members [IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F]. IL-17 family members are involved in numerous immune regulatory functions, including inducing and mediating proinflammatory responses and allergic responses. IL-17 induces the production of many other cytokines (IL-6, G-CSF, GM-CSF, IL-1beta, TGF-beta, and TNF-alpha), chemokines, including IL-8 (CXCL8), GRO-alpha (CXCL1) and MCP-1 (CCL2) and prostaglandins from many cell types (fibroblasts, endothelial cells, epithelial cells, keratinocytes and macrophages).
Reactivity - ELISA
Bovine IL-17A - Strong
Canine IL-17A - Weak
Equine IL-17A - Strong
Human IL-17A - Weak
Mouse IL-17A - Weak
Ovine IL-17A - Strong
Rabbit IL-17A - Weak
Rat IL-17A - Moderate
Swine IL-17A - Strong
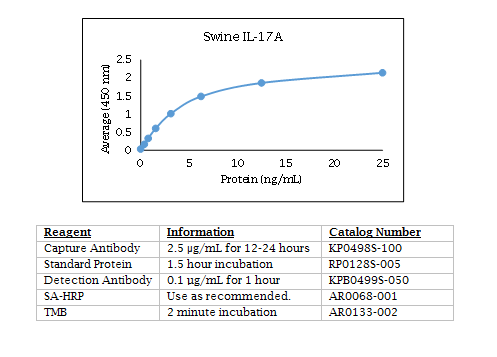
Swine IL-17A ELISA Data
Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge.
Magiri RB, Lai KJ, Mutwiri GK, Wilson HL.
Vaccines (Basel). 2020 May 18;8(2):E235. doi: 10.3390/vaccines8020235.
Applications: Enumeration of swine IL-17A and IL-13 release from lymph node cells by ELISPOT
Abstract
Vaccination is the most efficient method of protection against influenza infections. However, the rapidly mutating viruses and development of new strains make it necessary to develop new influenza vaccines annually. Hence, vaccines that stimulate cross-protection against multiple influenza subtypes are highly sought. Recent evidence suggests that adjuvants such as PCEP that promote Th1-type T cell and Th2-type T cell immune responses and broad-spectrum immune responses may confer cross-protection against heterologous influenza strains. In this study, we evaluated whether the immunogenic and protective potential of PCEP-adjuvanted inactivated swine influenza virus H1N1 vaccine can protect pigs immunized against live H3N2 virus. Piglets were vaccinated via the intradermal route with PCEP-adjuvanted inactivated swine influenza virus (SIV) H1N1 vaccine, boosted at day 21 with the same vaccines then challenged with infectious SIV H3N2 virus at day 35 via the tracheobronchial route. The pigs showed significant anti-H1N1 SIV specific antibody titres and H1N1 SIV neutralizing antibody titres, and these serum titres remained after the challenge with the H3N2 virus. In contrast, vaccination with anti-H1N1 SIV did not trigger anti-H3N2 SIV antibody titres or neutralizing antibody titres and these titres remained low until pigs were challenged with H3N2 SIV. At necropsy (six days after challenge), we collected prescapular lymph nodes and tracheobronchial draining the vaccination sites and challenge site, respectively. ELISPOTs from lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significant production of IFN-γ in the tracheobronchial cells, but not the prescapular lymph nodes. In contrast, lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significantly higher IL-13 and IL-17A in the prescapular lymph nodes draining the vaccination sites relative to unchallenged animals. Lung lesion scores show that intradermal vaccination with H1N1 SIV plus PCEP did not prevent lesions when the animals were challenged with H3N2. These results confirm previous findings that PCEP is effective as a vaccine adjuvant in that it induces strong immune responses and protects against homologous swine influenza H1N1 virus, but the experimental H1N1 vaccine failed to cross-protect against heterologous H3N2 virus.
The Cheese Matrix Modulates the Immunomodulatory Properties of Propionibacterium freudenreichii CIRM-BIA 129 in Healthy Piglets.
Rabah H, Ferret-Bernard S, Huang S, Le Normand L, Cousin FJ, Gaucher F, Jeantet R, Boudry G, Jan G.
Front Microbiol. 2018 Oct 29;9:2584. doi: 10.3389/fmicb.2018.02584. eCollection 2018.
Applications: Measurement of swine IL-17A culture supernatants of PBMC and MLNC by ELISA
Abstract
Propionibacterium freudenreichii is a beneficial bacterium, used as a cheese starter, which presents versatile probiotic properties. These properties are strain-dependent. We hypothesized they may also be delivery vehicle-dependent. In this study, we thus explored in healthy piglets how the cheese matrix affects the immunomodulatory properties of P. freudenreichii. During 2 weeks, three groups of weaned piglets consumed, respectively, P. freudenreichii as a liquid culture (PF-culture), P. freudenreichii under the form of a cheese (PF-cheese), or a control sterile cheese matrix (Cheese-matrix). The in vivo metabolic activity of P. freudenreichii was assessed by determining short chain fatty acids (SCFA) concentration and bifidobacteria population in feces. Whatever the delivery vehicle, P. freudenreichii was metabolically active in piglets' colon and enhanced both bifidobacteria and SCFA in feces. P. freudenreichii consumption decreased the secretion of TNFα and of IL-10 by peripheral blood mononuclear cells (PBMC). It did not alter IL-10, IFNγ, IL-17, and TNFα secretion in mesenteric lymph node immune cells (MLNC). PF-cheese enhanced significantly Treg phenotype, while PF-culture decreased significantly Th17 phenotype in PBMC and MLNC. Remarkably, only PF-cheese induced an increase of Th2 phenotype in PBMC and MLNC. Ex vivo stimulation of PBMC and MLNC by Lipopolysaccharides and Concanavalin A emphasized the difference in the immunomodulatory responses between PF-culture and PF-cheese group, as well as between PBMC and MLNC. This study shows the importance to consider the delivery vehicle for probiotic administration. It confirms the anti-inflammatory potential of P. freudenreichii. It opens new perspectives for the use propionibacteria-fermented products as preventive agents for inflammatory bowel diseases and intestinal infectious diseases.
Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of Th17-mediated response.
Cano PM, Seeboth J, Meurens F, Cognie J, Abrami R, Oswald IP, Guzylack-Piriou L.
PLoS One. 2013;8(1):e53647. doi: 10.1371/journal.pone.0053647. Epub 2013 Jan 10.
Applications: Measurement of swine IL-17A in cell culture supernatants by ELISA
Abstract
BACKGROUND/AIMS:
Deoxynivalenol (DON) is a mycotoxin produced by Fusarium species which is commonly found in temperate regions worldwide as a natural contaminant of cereals. It is of great concern not only in terms of economic losses but also in terms of animal and public health. The digestive tract is the first and main target of this food contaminant and it represents a major site of immune tolerance. A finely tuned cross-talk between the innate and the adaptive immune systems ensures the homeostatic equilibrium between the mucosal immune system and commensal microorganisms. The aim of this study was to analyze the impact of DON on the intestinal immune response.
METHODOLOGY:
Non-transformed intestinal porcine epithelial cells IPEC-1 and porcine jejunal explants were used to investigate the effect of DON on the intestinal immune response and the modulation of naive T cells differentiation. Transcriptomic proteomic and flow cytometry analysis were performed.
RESULTS:
DON induced a pro-inflammatory response with a significant increase of expression of mRNA encoding for IL-8, IL-1α and IL-1β, TNF-α in all used models. Additionally, DON significantly induced the expression of genes involved in the differentiation of Th17 cells (STAT3, IL-17A, IL-6, IL-1β) at the expenses of the pathway of regulatory T cells (Treg) (FoxP3, RALDH1). DON also induced genes related to the pathogenic Th17 cells subset such as IL-23A, IL-22 and IL-21 and not genes related to the regulatory Th17 cells (rTh17) such as TGF-β and IL-10.
CONCLUSION:
DON triggered multiple immune modulatory effects which could be associated with an increased susceptibility to intestinal inflammatory diseases.
Changes in Glomerular Filtration Rate After Renal Revascularization Correlate With Microvascular Hemodynamics and Inflammation in Swine Renal Artery Stenosis.
Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO.
Circ Cardiovasc Interv. 2012 Oct 9.
Applications: Measurement of Swine MCP-1 (CCL2), IFN gamma, and IL-17A in plasma by ELISA.
BACKGROUND:
The selection of patients with renal artery stenosis (RAS) likely to improve glomerular filtration rate (GFR) after percutaneous transluminal renal angioplasty is difficult. We examined basal hemodynamic and inflammatory factors linked to improved stenotic kidney (STK) function after percutaneous transluminal renal angioplasty in swine RAS.
METHODS AND RESULTS:
Fifteen pigs after 6 weeks of hemodynamically significant RAS were studied before and 4 weeks after technically successful percutaneous transluminal renal angioplasty+stenting. STK and contralateral kidney hemodynamics and function were evaluated by multidetector computed-tomography before and after acetylcholine challenge. Single-kidney deoxyhemoglobin (R2*, reciprocal to blood relaxation) and energy-dependent tubular function were assessed using blood-oxygen-level-dependent magnetic resonance imaging before and after furosemide. Baseline renal vein and inferior vena cava levels of inflammatory markers were measured and their gradient and net release calculated. Baseline parameters were compared with normal (n=7) and sham-RAS (n=7) pigs and correlated with the change in STK-GFR after revascularization (ΔGFR). Four weeks after percutaneous transluminal, renal angioplasty blood pressure was normalized in all animals, but STK-GFR improved in 10 of 15 (ΔGFR =+22.0±8.5 mL/min). ΔGFR correlated inversely with basal STK-GFR, renal release of inflammatory markers, and medullary R2* response to furosemide, but directly with GFR response to acetylcholine. Basal contralateral kidney GFR correlated directly with ΔGFR.
CONCLUSIONS:
Low basal STK-GFR with preserved response to acetylcholine may predict benefit from revascularization in RAS, whereas renal inflammation and robust STK-R2* responses to furosemide (possibly reflecting avid tubular oxygen consumption) are associated with less favorable outcomes. These tools may be useful for identification of patients likely to improve renal function after revascularization.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart