Chicken IL-2 Do-It-Yourself ELISA, ≤10 Plates
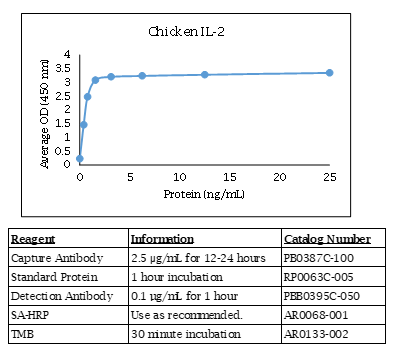
Chicken IL-2 ELISA Data
Chicken IL-2 ELISA Kit Components
| Component | Usage | Quantity | Catalog # |
| Anti-Chicken IL-2 Polyclonal Antibody | Capture Antibody | 100 µg | PB0387C-100 |
| Biotinylated Anti-Chicken IL-2 Polyclonal Antibody | Detection Antibody | 50 µg | PBB0395C-050 |
| Chicken IL-2 Recombinant Protein Standard | Standard | 5 µg | RP0063C-005 |
Specifications
The Chicken IL-2 Do-It-Yourself ELISA contains capture antibody, standard, and detection antibody for development of a Chicken IL-2 ELISA. The antibodies have been determined to function in an ELISA with the standard provided. Optimal buffers, concentrations, incubation times, incubation temperatures, and methods for the ELISA have not been determined. A working knowledge of ELISA is strongly recommended. The quantities of components provided are not matched. Components may also be purchased separately.
For additional tips and techniques to ensure a successful ELISA, check out our ELISA Technical Guide.
Background
Interleukin-2 (IL-2) is a cytokine produced by T-helper cells in response to antigenic or mitogenic stimulation. It is required for T-cell proliferation and other activities crucial to the regulation of the immune response. IL-2 was discovered to be a member of a family of cytokines, which also includes IL-4, IL-7, IL-9, IL-15 and IL-21. IL-2 signals through a receptor complex consisting of IL-2 specific IL-2 receptor alpha (CD25), IL-2 receptor beta (CD122) and a common gamma chain (γc). All members of this family use the common gamma chain as part of their signaling complex.
Alternate Names - IL2, IL-2, TCGF, lymphokine, interleukin 2
Chlamydia psittaci PmpD-N Exacerbated Chicken Macrophage Function by Triggering Th2 Polarization and the TLR2/MyD88/NF-κB Signaling Pathway
Chu J, Li X, Qu G, Wang Y, Li Q, Guo Y, Hou L, Liu J, Eko FO, He C.
Int J Mol Sci. 2020 Mar 15;21(6):2003. doi: 10.3390/ijms21062003.
Applications: Measurement of chicken IL-2, IL-6, IL-10, IL-12, and IFN-γ in cell culture supernatants by ELISA
Abstract
The polymorphic membrane protein D (PmpD) is a highly conserved outer membrane protein which plays an important role in pathogenesis during Chlamydia psittaci infection. In this study, we evaluated the ability of the N-terminus of PmpD (PmpD-N) to modulate the functions of chicken macrophages and the signaling pathway(s) involved in PmpD-N-induced Toll-like receptors (TLRs), as well as interleukin (IL)-6 and IL-10 cytokine secretions. Thus, HD11 macrophages were treated with exogenous and intracellular PmpD-N of C. psittaci. The chlamydial growth was evaluated by enumeration of chlamydial loads in the infected macrophages. The phagocytic function of macrophages following PmpD-N treatment was detected by fluorescein-labeled Escherichia coli (E. coli). The concentration of nitric oxide (NO) secreted by HD11 macrophages was measured by the amount of NO2- in the culture supernatant using the Griess method. The cytokine secretions were assessed using multiplex cytokine ELISA kits. Expression levels of TLRs, myeloid differentiation factor 88 (MyD88), and nuclear factor kappa B (NF-κB) were analyzed by a Western blotting assay, as well as a luciferase assay, while NF-κB p65 nuclear translocation was assessed by confocal microscopy. The nuclear translocation of the transcription factor NF-κB was confirmed by evaluating its ability to combine with the corresponding promoter using the electrophoretic mobility shift assay (EMSA). After treatment with exogenous or endogenous PmpD-N, chlamydial loads and phagocytic functions were reduced significantly compared with those of the plasmid vector group, while NO secretions were reduced significantly compared with those of the lipopolysaccharide (LPS) treatment. Stimulation of HD11 cells with PmpD-N provoked the secretion of the Th2 cytokines, IL-6, and IL-10 and upregulated the expression of TLR2, TLR4, MyD88, and NF-κB. Furthermore, inhibition of TLR2, MyD88, and NF-κB in HD11 cells significantly decreased IL-6 and IL-10 cytokine levels, while NO production and phagocytosis increased significantly, strongly suggesting their involvement in PmpD-N-induced Th2 cytokine secretion and macrophage dysfunction. Our data indicate that C. psittaci PmpD-N inhibited macrophage functions by activating the Th2 immune response and the TLR2/MyD88/NF-κB signaling pathway.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart