Swine IFN gamma Do-It-Yourself ELISA, ≤20 Plates
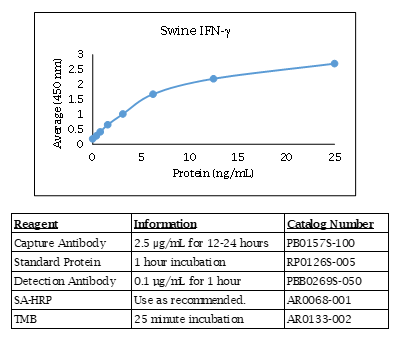
Swine IFN-γ ELISA Data
Swine IFN-γ ELISA Kit Components
| Component | Usage | Quantity | Catalog # |
| Anti-Swine IFNγ Polyclonal Antibody | Capture Antibody | 100 µg X 2 | PB0157S-100 |
| Biotinylated Anti-Swine IFNγ Polyclonal Antibody | Detection Antibody | 50 µg | PBB0269S-050 |
| Swine IFNγ Recombinant Protein | Standard | 5 µg | RP0126S-005 |
Swine IFN-γ ELISA Specifications
The Swine IFNγ Do-It-Yourself ELISA contains capture antibody, standard, and detection antibody for development of a Swine IFNγ ELISA. The antibodies have been determined to function in an ELISA with the standard provided. Optimal buffers, concentrations, incubation times, incubation temperatures, and methods for the ELISA have not been determined. A working knowledge of ELISA is strongly recommended. The quantities of components provided are not matched. Components may also be purchased separately.
For additional tips and techniques to ensure a successful ELISA, check out our ELISA Technical Guide.
IFN-γ Background
Interferon-gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. This interferon was originally called macrophage-activating factor, a term now used to describe a larger family of proteins to which IFN-γ belongs. IFN-γ, or type II interferon, is a cytokine that is critical for innate and adaptive immunity against viral and intracellular bacterial infections and for tumor control. Aberrant IFN-γ expression is associated with a number of autoinflammatory and autoimmune diseases. The importance of IFN-γ in the immune system stems in part from its ability to inhibit viral replication directly, but, most important, derives from its immunostimulatory and immunomodulatory effects. IFN-γ is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once antigen-specific immunity develops.
Alternate Names - IFNG, IFG, IFI, interferon, gamma, interferon gamma
Changes in Glomerular Filtration Rate After Renal Revascularization Correlate With Microvascular Hemodynamics and Inflammation in Swine Renal Artery Stenosis.
Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO.
Circ Cardiovasc Interv. 2012 Oct 9.
Applications: Measurement of Swine MCP-1 (CCL2), IFN gamma, and IL-17A in plasma by ELISA.
BACKGROUND:
The selection of patients with renal artery stenosis (RAS) likely to improve glomerular filtration rate (GFR) after percutaneous transluminal renal angioplasty is difficult. We examined basal hemodynamic and inflammatory factors linked to improved stenotic kidney (STK) function after percutaneous transluminal renal angioplasty in swine RAS.
METHODS AND RESULTS:
Fifteen pigs after 6 weeks of hemodynamically significant RAS were studied before and 4 weeks after technically successful percutaneous transluminal renal angioplasty+stenting. STK and contralateral kidney hemodynamics and function were evaluated by multidetector computed-tomography before and after acetylcholine challenge. Single-kidney deoxyhemoglobin (R2*, reciprocal to blood relaxation) and energy-dependent tubular function were assessed using blood-oxygen-level-dependent magnetic resonance imaging before and after furosemide. Baseline renal vein and inferior vena cava levels of inflammatory markers were measured and their gradient and net release calculated. Baseline parameters were compared with normal (n=7) and sham-RAS (n=7) pigs and correlated with the change in STK-GFR after revascularization (ΔGFR). Four weeks after percutaneous transluminal, renal angioplasty blood pressure was normalized in all animals, but STK-GFR improved in 10 of 15 (ΔGFR =+22.0±8.5 mL/min). ΔGFR correlated inversely with basal STK-GFR, renal release of inflammatory markers, and medullary R2* response to furosemide, but directly with GFR response to acetylcholine. Basal contralateral kidney GFR correlated directly with ΔGFR.
CONCLUSIONS:
Low basal STK-GFR with preserved response to acetylcholine may predict benefit from revascularization in RAS, whereas renal inflammation and robust STK-R2* responses to furosemide (possibly reflecting avid tubular oxygen consumption) are associated with less favorable outcomes. These tools may be useful for identification of patients likely to improve renal function after revascularization.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart