Bovine VEGF-A Polyclonal Antibody
Vascular endothelial growth factor (VEGF) proteins stimulate vasculogenesis and angiogenesis. They are part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. The normal function of VEGF proteins is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. The VEGF family has six members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and Placental Growth Factor (PGF). Activity of VEGF-A, as its name implies, has been studied mostly on cells of the vascular endothelium, although it does have effects on a number of other cell types (e.g., stimulation monocyte/macrophage migration, neurons, cancer cells, kidney epithelial cells). In vitro, VEGF-A has been shown to stimulate endothelial cell mitogenesis and cell migration. VEGF-A is also a vasodilator and increases microvascular permeability and was originally referred to as vascular permeability factor (VPF).
Reactivity - ELISA
Bovine VEGF-A - Strong
Canine VEGF-A - Strong
Equine VEGF-A - Strong
Feline VEGF-A - Strong
Mouse VEGF-A - Strong
Ovine VEGF-A - Strong
Rabbit VEGF-A - Weak
Rabbit VEGF-A (165) - Weak
Rat VEGF-A - Strong
Swine VEGF-A - Strong
Zebrafish VEGF-A - None
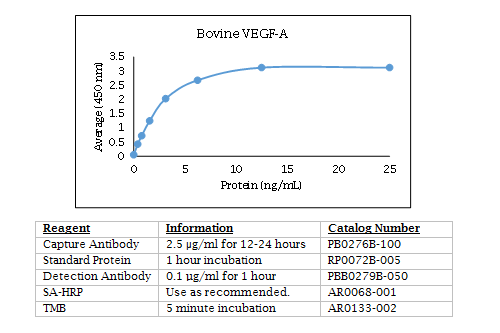
Bovine VEGF-A ELISA Data
Effect of ovarian steroids on vascular endothelial growth factor a expression in bovine uterine endothelial cells during adenomyosis.
Lupicka M, Zadroga A, Szczepańska A, Korzekwa AJ.
BMC Vet Res. 2019 Dec 30;15(1):473. doi: 10.1186/s12917-019-2222-0.
Applications: Measurement of bovine VEGF-A in culture media by ELISA
Abstract
BACKGROUND:
Adenomyosis is a uterine dysfunction defined as the presence of endometrial glands within the myometrium. There is evidence that proangiogenic factors may play a role during the development of adenomyosis; however, exact mechanism remains unknown. The aim of the study was to determine the action of vascular endothelial growth factor A (VEGFA) in uterine tissue and uterine vascular endothelial cells during adenomyosis.
RESULTS:
Uterine tissues were collected and examined for the presence and extent of adenomyosis. Gene and protein expression of VEGFA and its two receptors (VEGFR1 and VEGFR2) was evaluated with quantitative polymerase chain reaction and Western blotting, respectively, in endometrium and myometrium during adenomyosis. Immunolocalization of VEGFA and its receptors within uterine tissues during adenomyosis was also determined. In an in vitro experiment, endothelial cells from non-adenomyotic bovine uteri were treated with media conditioned by non-adenomyotic or adenomyotic uterine slices treated with 17-beta-oestradiol (E2) or progesterone (P4). Both gene and protein expression of VEGFR2 were elevated in endometrium in stages 3-4 of adenomyosis. Protein expression of VEGFA and VEGFR2 as well as VEGFA secretion were increased in endothelial cells treated with media conditioned by adenomyotic uterine slices after E2 treatment.
CONCLUSIONS:
Results suggest that VEGFA signalling is an important component, next to E2, that enhances VEGFA action and participates in adenomyosis development in cows.
Ordering Information & Terms and Conditions
We require a phone number and e-mail address for both the end user of the ordered product and your institution's Accounts Payable representative. This information is only used to help with technical and billing issues.
Via Phone
Please call us at 651-646-0089 between the hours of 8:30 a.m. and 5:30 p.m. CST Mon - Fri.
Via Fax
Orders can be faxed to us 24 hours a day at 651-646-0095.
Via E-mail
Please e-mail orders to orders@KingfisherBiotech.com.
Via Mail
Please mail your order to:
Sales Order Entry
Kingfisher Biotech, Inc.
1000 Westgate Drive
Suite 123
Saint Paul, MN 55114
USA
Product Warranty
Kingfisher Biotech brand products are warranted by Kingfisher Biotech, Inc. to meet stated product specifications and to conform to label descriptions when used, handled and stored according to instructions. Unless otherwise stated, this warranty is limited to one year from date of sale. Kingfisher Biotech’s sole liability for the product is limited to replacement of the product or refund of the purchase price. Kingfisher Biotech brand products are supplied for research applications. They are not intended for medicinal, diagnostic or therapeutic use. The products may not be resold, modified for resale or used to manufacture commercial products without prior written approval from Kingfisher Biotech.
Payment Terms
All prices are subject to change without notice. Payment terms are net thirty (30) days from receipt of invoice. A 1.5% service charge per month is added for accounts past due over 30 days. Prices quoted are U.S. Dollars. The purchaser assumes responsibility for any applicable tax. You will only be charged for products shipped. Products placed on back order will be charged when shipped. If you place an order and fail to fulfill the terms of payment, Kingfisher Biotech, Inc. may without prejudice to any other lawful remedy defer further shipments and/or cancel any order. You shall be liable to Kingfisher Biotech, Inc. for all costs and fees, including attorneys' fees, which Kingfisher Biotech, Inc. may reasonably incur in any actions to collect on your overdue account. Kingfisher Biotech, Inc. does not agree to, and is not bound by, any other terms or conditions such as terms in a purchase order that have not been expressly agreed to in writing signed by a duly authorized officer of Kingfisher Biotech, Inc.
Shipping
Shipping and handling costs are prepaid and added to the invoice. Shipping and handling costs will be charged only on the first shipment in situations where an order contains back ordered products. Kingfisher Biotech, Inc. reserves the right to select the packaging and shipping method for your order, which will ensure the stability of the product and also efficient tracing. Domestic orders will normally be shipped by overnight. Damage during shipment is covered by the warranty provided in these terms and conditions. For international orders, title to the goods passes in the United States when the goods are placed with the shipper. For all orders, the risk of loss of the goods passes when the goods are placed with the shipper.
Returns
Please call customer service before returning any products for refund, credit or replacement. NO returns will be accepted without prior written authorization. Returns are subject to a restocking fee of 20%.



 New Products
New Products Ordering
Ordering Distributors
Distributors Resources
Resources FAQs
FAQs Cart
Cart